Russo, E; Citraro, R; Mula, M
(2017)
The preclinical discovery and development of brivaracetam for the treatment of focal epilepsy.
Expert Opin Drug Discov, 12 (11).
pp. 1169-1178.
ISSN 1746-045X
https://doi.org/10.1080/17460441.2017.1366985
SGUL Authors: Mula, Marco
|
PDF
Accepted Version
Available under License ["licenses_description_publisher" not defined]. Download (577kB) | Preview |
|
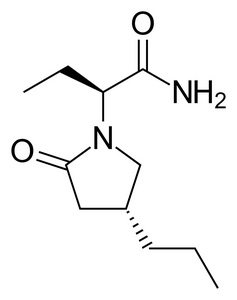
![[img]](https://openaccess.sgul.ac.uk/109223/9.hassmallThumbnailVersion/Figure%201.jpg)
|
Image (JPEG) (Figure 1)
Accepted Version
Available under License ["licenses_description_publisher" not defined]. Download (87kB) | Preview |
Abstract
INTRODUCTION: Brivaracetam (BRV) is a new AED currently licensed for the adjunctive treatment of adult patients with focal epilepsies. It is a ligand of the ubiquitous synaptic vesicle glycoprotein 2A (SV2A). Areas covered: This paper covers the preclinical and subsequent clinical development of BRV focusing on the discovery of the SV2A protein as the main target for levetiracetam (LEV) and the main similarities and differences between LEV and BRV in terms of pharmacodynamic and pharmacokinetic properties. Phase II and Phase III studies are also presented and data from post-marketing phase IV studies are discussed. Expert opinion: The preclinical development of BRV is quite unique and has raised several doubts on current methodologies adopted for AED development, reinforcing the need for new approaches. The preclinical and clinical profile suggest that BRV is potentially an ideal compound in the emergency setting given the rapid onset of action associated with being water soluble and, therefore, available in intravenous formulation. In addition, data from Phase III studies have already suggested that BRV may be effective not only in focal epilepsies but also in generalised syndromes. Further data from special populations such as children and women of child bearing age are urgently needed.
| Item Type: | Article | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Additional Information: | This is an Accepted Manuscript of an article published by Taylor & Francis in Expert Opinion on Drug Discovery on 23/8/17, available online: http://www.tandfonline.com/10.1080/17460441.2017.1366985. | ||||||||
| Keywords: | Brivaracetam, SV2A, antiepileptic drugs, epilepsy, levetiracetam, Pharmacology & Pharmacy | ||||||||
| SGUL Research Institute / Research Centre: | Academic Structure > Institute of Medical & Biomedical Education (IMBE) Academic Structure > Institute of Medical & Biomedical Education (IMBE) > Centre for Clinical Education (INMECE ) |
||||||||
| Journal or Publication Title: | Expert Opin Drug Discov | ||||||||
| ISSN: | 1746-045X | ||||||||
| Language: | eng | ||||||||
| Dates: |
|
||||||||
| Publisher License: | Publisher's own licence | ||||||||
| PubMed ID: | 28829199 | ||||||||
 |
Go to PubMed abstract | ||||||||
| URI: | https://openaccess.sgul.ac.uk/id/eprint/109223 | ||||||||
| Publisher's version: | https://doi.org/10.1080/17460441.2017.1366985 |
Statistics
Actions (login required)
 |
Edit Item |