Pansa, P; Hsia, Y; Bielicki, J; Lutsar, I; Walker, AS; Sharland, M; Folgori, L
(2018)
Evaluating safety reporting in paediatric antibiotic trials 2000-2016: a systematic review and meta-analysis.
Drugs, 78 (2).
pp. 231-244.
ISSN 1179-1950
https://doi.org/10.1007/s40265-017-0850-x
SGUL Authors: Hsia, Yingfen
|
Microsoft Word (.docx)
Accepted Version
Available under License ["licenses_description_publisher" not defined]. Download (100kB) |
||
|
Microsoft Word (.docx) (Supplementary figures)
Accepted Version
Available under License ["licenses_description_publisher" not defined]. Download (349kB) |
||
|
Microsoft Word (.docx) (Supplementary material)
Accepted Version
Available under License ["licenses_description_publisher" not defined]. Download (54kB) |
||
![[img]](https://openaccess.sgul.ac.uk/109320/7.hassmallThumbnailVersion/Fig%201.jpg)
|
Image (JPEG) (Figure 1)
Accepted Version
Available under License ["licenses_description_publisher" not defined]. Download (89kB) | Preview |
|
![[img]](https://openaccess.sgul.ac.uk/109320/12.hassmallThumbnailVersion/Fig%202.jpg)
|
Image (JPEG) (Figure 2)
Accepted Version
Available under License ["licenses_description_publisher" not defined]. Download (75kB) | Preview |
|
![[img]](https://openaccess.sgul.ac.uk/109320/19.hassmallThumbnailVersion/Fig%203.jpg)
|
Image (JPEG) (Figure 3)
Accepted Version
Available under License ["licenses_description_publisher" not defined]. Download (136kB) | Preview |
Abstract
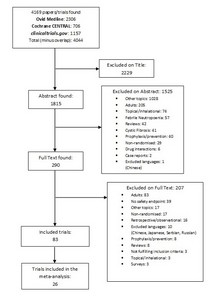
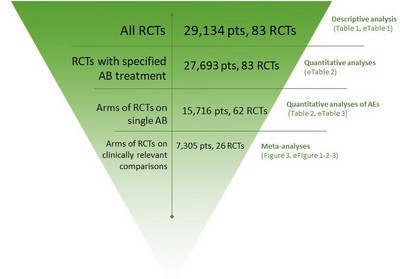
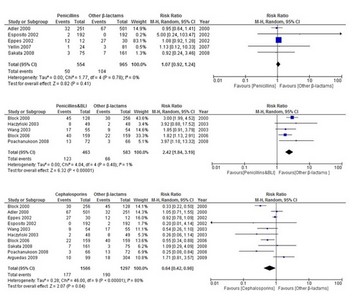
Background There are very few options to treat multidrug-resistant bacterial infections in children. A major barrier is the duration and complexity of regulatory trials of new antibiotics. Extrapolation of safety data from adult trials could facilitate drug development for children. Objective We performed a systematic review on the safety of antibiotic clinical trials (CTs) in children (0–18 years) to evaluate the overall quality of safety trials conducted in children and to determine if age-specific adverse events (AEs) could be identified for specific antibiotic classes. Data Sources We searched the MEDLINE, Cochrane CENTRAL, and ClinicalTrials.gov electronic databases for trials conducted between 2000 and 2016. Study Selection All trials in which safety was declared a primary or secondary endpoint were included. Exclusion criteria were (1) topical or inhalational route of administration; (2) non-infectious conditions; (3) administration for prophylaxis rather than treatment; (4) selected population (i.e. cystic fibrosis, malignancies, HIV and tuberculosis); and (5) design other than randomized controlled trials. Trials reporting data on both adults and children were included only if paediatric results were reported separately. Data Extraction and Synthesis Two authors independently extracted the data. To assess the quality of published trials, the Extension for harms for Consolidated Standards of Reporting Trials (CONSORT) Statement 2004 was used. Main Outcome and Measure In order to quantitatively assess the rate of developing AEs by drug class, the numbers of overall and body-system-specific AEs were collected for each study arm, and then calculated per single drug class as median and interquartile range (IQR) of the proportions across CTs. The AEs most frequently reported were compared in the meta-analysis by selecting the CTs on the most represented drug classes. Results Eighty-three CTs were included, accounting for 27,693 children. Overall, 69.7% of CONSORT items were fully reported. The median proportion of children with any AE was 22.5%, but did not exceed 8% in any single body system. Serious drug-related AEs and drug-related discontinuations were very rare (median 0.3 and 0.9%, respectively). Limitations included the inability to stratify by age group, particularly neonates. Conclusions and Relevance Overall, AEs in paediatric antibiotic CTs were predictable and class-specific, and no unexpected (age-specific) side effects were identified. Smaller, open-label, dose-finding, high-quality, single-arm pharmacokinetic trials seem potentially sufficient for certain common antibiotic classes, extrapolating well-established safety profiles determined from large adult efficacy trials. This approach could reduce duration and enhance subsequent registration of urgently needed new antibiotics. This will need to be combined with enhanced methods of pharmacovigilance for monitoring of emerging AEs in routine clinical practice.
| Item Type: | Article | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Additional Information: | This is a post-peer-review, pre-copyedit version of an article published in Drugs. The final authenticated version is available online at: http://dx.doi.org/10.1007/s40265-017-0850-x | ||||||||
| SGUL Research Institute / Research Centre: | Academic Structure > Infection and Immunity Research Institute (INII) | ||||||||
| Journal or Publication Title: | Drugs | ||||||||
| ISSN: | 1179-1950 | ||||||||
| Dates: |
|
||||||||
| Publisher License: | Publisher's own licence | ||||||||
| URI: | https://openaccess.sgul.ac.uk/id/eprint/109320 | ||||||||
| Publisher's version: | https://doi.org/10.1007/s40265-017-0850-x |
Statistics
Actions (login required)
 |
Edit Item |